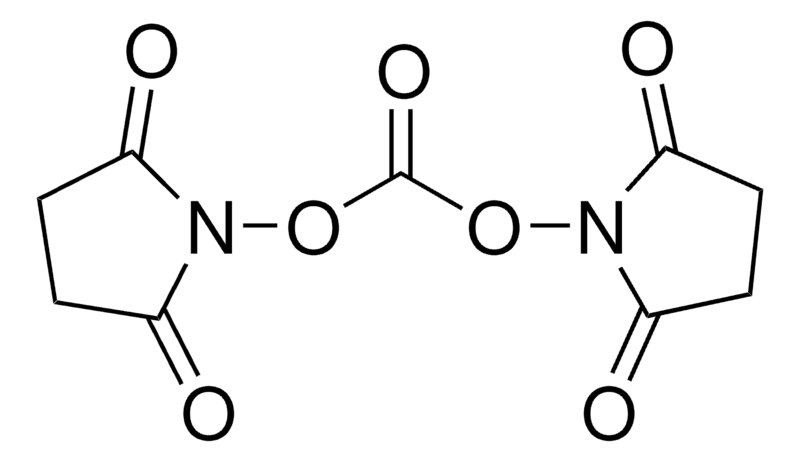
N,N’-Disuccinimidyl Carbonate (DSC) is a versatile reagent used for the preparation of N-succinimidyl esters of N-protected amino acids and other carboxylic acids, facilitating peptide synthesis and bioconjugation. As an activated carbonate, DSC is employed in synthesizing ureas and carbamates. Additionally, it is widely used for the coupling of ligands to peptides via lysine residues.
CAS: 74124-79-1
Synonym:Di(N-succinimidyl) carbonate N-Succinimidyl carbonate
Properties
Purity
≥98.0%
Molecular Formula
C9H8N2O7
Molecular Weight
256.17 [gr/mol]
Appearance
White to off-white crystalline powder
Storage Conditions
Store in a cool and dry place (2-8oC)
Applications
- Preparation of N-succinimidyl esters of N-protected amino acids and other carboxylic acids, useful in peptide synthesis.
- Activation of hydroxyl and amine groups in organic synthesis.
- Synthesis of ureas and carbamates, offering a mild alternative to phosgene-based methods.
- Coupling of ligands to proteins via lysine residues for bioconjugation and drug delivery applications.
- Functionalization of polymers and surfaces, enhancing biomaterial applications.
- Crosslinking of biomolecules, such as proteins and peptides, in biochemical and pharmaceutical research
Studies
- Recent development in peptide coupling reagents
T. I. Al-Warhi, H. M.A. Al-Hazimi, and A. El-Faham Journal of Saudi Chemical Society, 2012, 16, 97–116.
Read Article - H. Ogura, T. Kobayashi, K. Shimizu, K. Kawabe, K. Takeda, “A novel active ester synthesis reagent (N,N’-disuccinimidyl carbonate),” Tetrahedron Lett., 1979, 20(49), 4745-4748. DOI: 10.1016/S0040-4039(01)86699-5
- G.T. Hermanson, Bioconjugate Techniques, 3rd ed., Academic Press, 2013. ISBN: 978-0-12-382239-0.
- A.K. Ghosh, T.T. Duong, S.P. McKee, W.J. Thompson, “N,N’-Disuccinimidyl Carbonate: A Useful Reagent for Alkoxycarbonylation of Amines,” Tetrahedron Lett., 1992, 33(19), 2787-2790. DOI: 10.1016/S0040-4039(00)78856-3