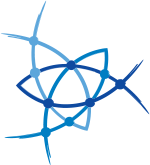
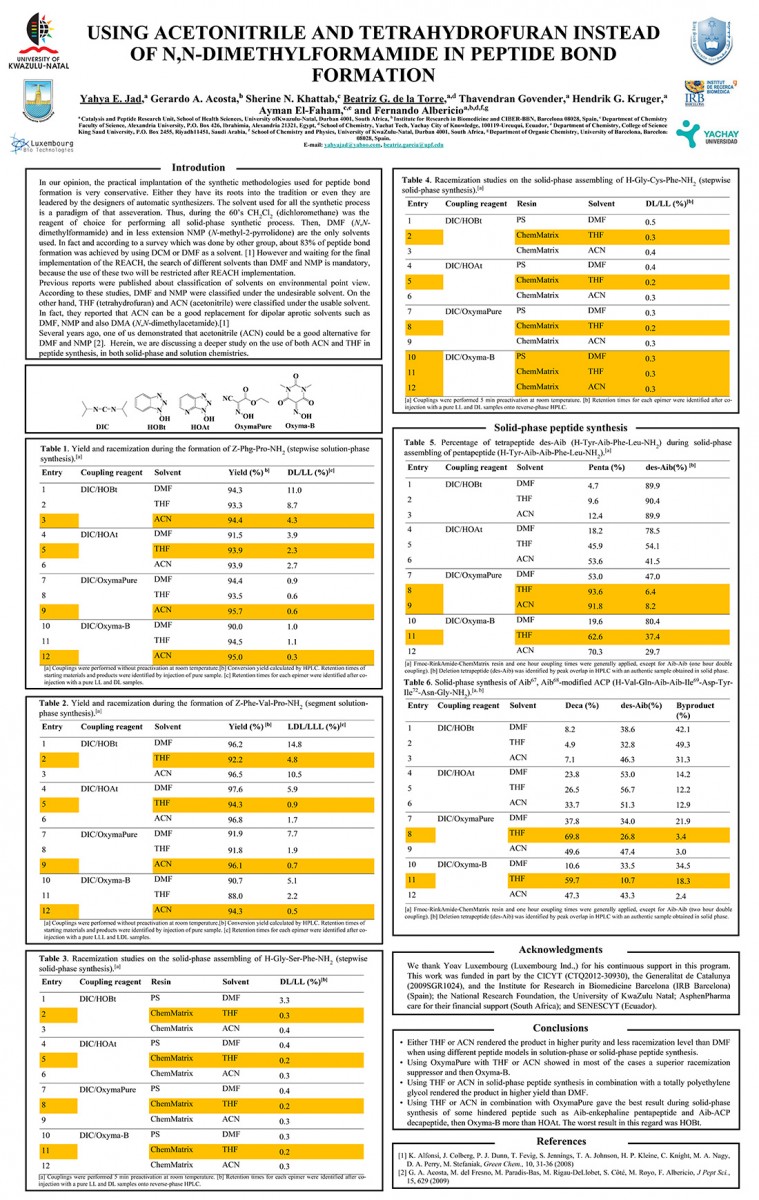
Oxy-B® is an oxime derived from 1,3-dimethylbarbituric acid, with a special orientation of the carbonyl moiety, and is one of the best oxime additives currently available for racemization-free peptide synthesis. Its ability to preserve chiral purity in peptide coupling reactions exceeds that of both OxymaPure® and HOAt.
CAS: 5417-13-0
Synonym: 5-(hydroxyimino)-1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione, DMVA
Properties
Purity
≥99.0%
Molecular Formula
C6H7N3O4
Molecular Weight
185.1 [gr/mol]
Appearance
White powder
Storage Conditions
Store in a cool and dry place
Applications
- Oxy-B can play an assisted basic catalytic role by enhancing the nucleophilicity of the amino function during coupling. In addition, Oxy-B does not show any ester moiety in its structure and therefore, there is no risk of any side-reaction.
- Oxy-B is soluble in a wide range of solvents, including green solvents such 2-methyl tetrahydrofuran (2-MeTHF) and cyclopentyl methyl ether (CPME).
- The additive Oxy-B can be used in the presence of carbodiimides and has been shown to be effective in the synthesis of difficult peptide sequences, with yields comparable to syntheses performed with OxymaPure®.
- The reaction can be monitored through the change in the color of the
solution. The solution is blue at the start of the reaction and after completion becomes a yellowish green. - Oxy-B does not contain any ethyl ester, which could lead to side reactions.
- Oxy-B presents a satisfactory performance in assembling demanding sequences such as the Aib-enkephalin pentapeptide (H-Tyr-Aib-Aib-Phe-Leu-NH2).
- Oxy-B showed better results than OxymaPure when used as a racemization suppressor and exceeded those achieved with HOAt in both stepwise and segment coupling in solid- and solution-phase peptide synthesis.
Studies
- Oxyma-B, an excellent racemization suppressor for peptide synthesis
Y. E. Jad, S. N. Khattab, B.G. de la Torre,T. Govender, H. G. Kruger, A. El-Fahamb, and F. Albericio Org. Biomol. Chem. 2014, 12, 8379–8385.
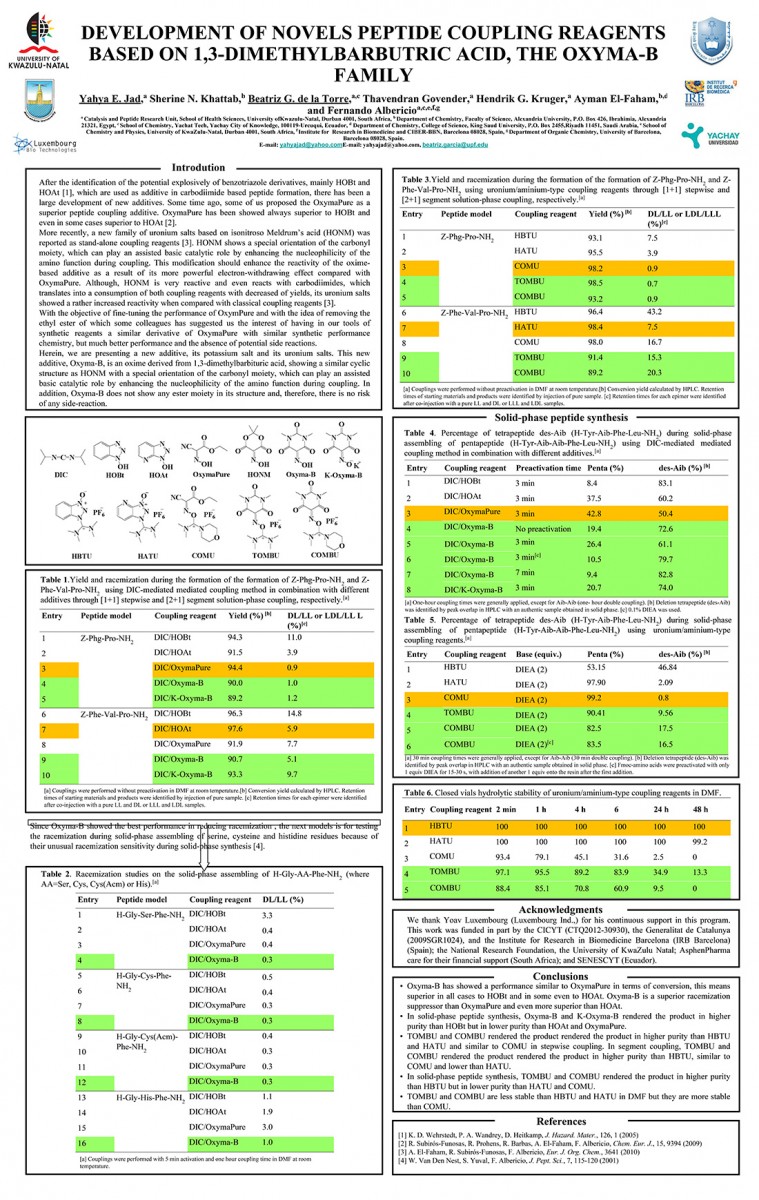
Read Article - Peptide Synthesis Beyond DMF:THF and ACN as Excellent and Friendlier Alternatives
Y. E. Jad, G. A. Acosta, S. N. Khattab, B. G. de la Torre, T. Govender, H. G. Kruger, A. El-Faham and F. Albericio, Org. Biomol. Chem., 2015,13, 2393-2398. - Read Article
- Carbodiimide-Mediated Beckmann Rearrangement of Oxyma-B as a Side Reaction in Peptide Synthesis Orlandin A, Guryanov I, Ferrazzano L, Biondi B, Biscaglia F, Storti C, Rancan M, Formaggio F, Ricci A, Cabri W. . Molecules. 2022 Jun 30;27(13):4235. doi: 10.3390/molecules27134235.
Posters
Development of Novels Peptide Coupling Reagents Based on 1,3-Dimethylbarbituric Acid, the Oxyma-B FAMILY
Yahya E. Jad, Sherine N. Khattab, Beatriz G. de la Torre, Thavendran Govender, Hendrik G. Kruger, Ayman El-Faham, and Fernando Albericio
View PosterUsing Acetonitrile and Tetrahydrofuran Instead of N,N-Dimethylformamide in Peptide Bond Formation
Yahya E. Jad, Gerardo A. Acosta, Sherine N. Khattab, Beatriz G. de la Torre, Thavendran Govender, Hendrik G. Kruger, Ayman El-Faham, and Fernando Albericio
View Poster