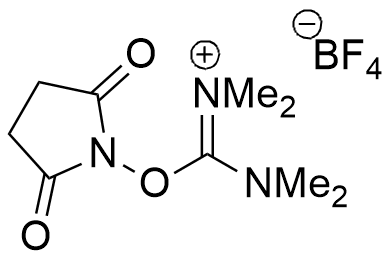
TSTU is a peptide coupling reagent converts carboxylates to N-succinimidyl active esters.
CAS: 105832-38-0
Synonym: N,N,N′,N′-Tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate
Properties
Purity
≥98.0%
Molecular Formula
C9H16BF4N3O3
Molecular Weight
301.05 [gr/mol]
Appearance
White or off-white powder
Storage Conditions
Store in cool place
Applications
- TSTU is used in intramolecular cyclization of 2-acyl-benzoic acids mediated, leading to diversely substituted (Z)-3-ylidenephthalides. The application of the method is highlighted by gram-scale preparation of the antiplatelet drug n-butylphthalide.
- TSTU, HOSu, and CuCl2 have been applied as coupling cocktail, proved to completely eliminate the racemization of the carboxy-terminal N-methylamino acid residue during segment condensation.
- Polymer-TSTU and Polymer-HSTU have been prepared from polymeric Nhydroxysuccinimide (P-HOSu) and used as solid-supported reagents for peptide coupling reactions.
Studies
- Transition-metal-free synthesis of (Z)-3-ylidenephthalides from 2-acyl-benzoic acids
B. H. Xinhua and X. Fengtian Tet. Lett., 2014, 55(11), 1956-1958. - O-(N-Succinimidyl)-1,1,3,3-tetramethyluronium Tetrafluoroborate– N-Hydroxysuccinimide–CuCl2: A Facile and Reliable System for Racemization-Free Coupling of Peptides Having a Carboxyterminal N-Methylamino Acid
Y. Nishiyama, S. Ishizuka, T. Mori, and K. Kurita Chem. Pharm. Bull., 2000, 48(3), 442-444. - Uronium salts from polymeric N-hydroxysuccinimide (P-HOSu) as new solid-supported peptide coupling reagents
R. Chinchilla, D. J. Dodsworth, C. Nájera, J. M. Soriano, and M. Yus ARKIVOC (Gainesville, FL, United States), 2003, 10, 41-47. - Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing
Jeffrey B. Sperry*, Christopher J. Minteer, JingYa Tao, Rebecca Johnson, Remzi Duzguner, Michael Hawksworth, Samantha Oke, Paul F. Richardson, Richard Barnhart, David R. Bill, Robert A. Giusto, and John D. Weaver III
Org. Process Res. Dev. 2018, 22, 9, 1262–1275