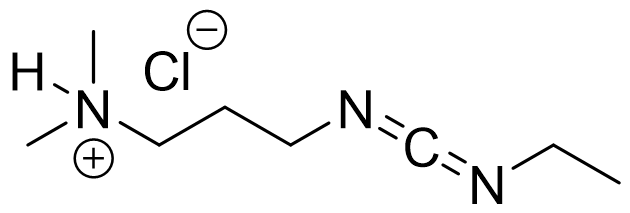
EDC·HCl, usually known as water soluble carbodiimide, is a versatile modern coupling agent.
CAS: 25952-53-8
Synonym: 1-Ethyl-3-(3-dimethyllaminopropyl)carbodiimide hydrochloride
Properties
Purity
≥98.0%
Molecular Formula
C8H17N3.HCl
Molecular Weight
191.7 [gr/mol]
Appearance
White to off-white crystalline powder
Storage Conditions
Store in a cool place
Applications
- It is an easily handled solid with high solubility in water (>200gl) and organic solvents such as dichloromethane, tetrahydrofuran and dimethylformamide.
- EDC·HCl replaced N,N-Dicyclohexylcarbodiimide (DCC) in solution-phase peptide synthesis because its urea is highly soluble in aqueous solutions, and so is easily removed by an aqueous work-up.
- As well as being used for the synthesis of amides, EDC·HCl is also used as a coupling agent in the preparation of esters from carboxylic acids using dimethylaminopyridine as the catalyst.
- Combinations of EDC·HCl and potassium salts of oximes are new and unique coupling cocktails that could be used in particular for the formation of hindered peptides such as Aib-enkephaline pentapeptide.
Studies
- DC.HCl and Potassium Salts of Oxyma and Oxyma-B as Superior Coupling Cocktails for Peptide Synthesis
Y. E. Jad, S. N. Khattab, B. G. de la Torre, T. Govender, H. G. Kruger, A. El-Faham, and F. Albericio Eur. J. Org. Chem., 2015, 14, 3116–3120.
Read Article - Evaluation of alternative solvents in common amide coupling reactions: replacement of dichloromethane and N,N-dimethylformamide
D. S. MacMillan, J. Murray, H. F. Sneddon, C. Jamieson, and A. J. B. Watson Green Chem., 2013, 15, 596–600. - Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing
Jeffrey B. Sperry*, Christopher J. Minteer, JingYa Tao, Rebecca Johnson, Remzi Duzguner, Michael Hawksworth, Samantha Oke, Paul F. Richardson, Richard Barnhart, David R. Bill, Robert A. Giusto, and John D. Weaver III
Org. Process Res. Dev. 2018, 22, 9, 1262–1275