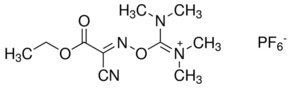
HOTU is a uronium coupling reagent that belongs to a family of OxymaPure® based coupling reagents.
The use of HOTU is possible for both solution synthesis and solid-phase peptide synthesis.
CAS: 333717-40-1
Synonym: O-[(Ethoxycarbonyl)cyanomethylenamino]-N,N,N′,N′-tetramethyluronium hexafluorophosphate
Properties
Purity
≥98.0%
Molecular Formula
C10H17F6N4O3P
Molecular Weight
386.23 [gr/mol]
Appearance
White to off-white crystalline powder
Storage Conditions
Protect from the light and store in a cool and dry place (≤25oC)
Applications
- HOTU was developed as a peptide coupling reagent by Luxembourg Bio-technologies Ltd, as an alternative to benzotriazole-based coupling reagents.
- HOTU is distinguished by its high activation potential, straightforward preparation (one-pot reaction), and high solubility.
- HOTU is the O-form isomer (uronium salts), which is more reactive than the typical hydroxybenzotriazole N-form (aminium salts).
- The by-products resulting from the activation of a carboxyl group with HOTU are tetramethylurea and ethyl 2-hydroxyimino-2-cyanoacetate, which can readily be extracted in the water.
- Moreover, the anion of the oxime is yellow in color and can therefore be readily detected on the extraction of the reaction mixture with, for example, bicarbonate. The use of HOTU is possible both in solution peptide synthesis and in solid-phase peptide synthesis.
Studies
- Azidopeptide nucleic acid. An alternative strategy for solid-phase peptide nucleic acid (PNA) synthesis)
F, Debaene and N. Winssinger Org. Lett., 2003, Vol. 5, No. 23, 4445-4447.
Read Article - Oxyma: an efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion
R. Subirós-Funosas, R. Prohens, R. Barbas, A. El-Faham, and F. Albericio Chemistry, 2009, 15(37), 9394-403.
Read Article - Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used in Pharmaceutical Manufacturing
Jeffrey B. Sperry*, Christopher J. Minteer, JingYa Tao, Rebecca Johnson, Remzi Duzguner, Michael Hawksworth, Samantha Oke, Paul F. Richardson, Richard Barnhart, David R. Bill, Robert A. Giusto, and John D. Weaver III
Org. Process Res. Dev. 2018, 22, 9, 1262–1275